Many people felt an initial sense of relief that the changes announced as part of the so called "RoHS2" proposals, back in December 2008, were not as widespread as feared. While new product categories (medical devices and monitoring and control instruments) will fall within scope, and it is likely that 4 new substances will be captured under RoHS (or REACH) and the separate review of exemptions will have some impact, the implementation dates of all three looked to be some way out in the future.
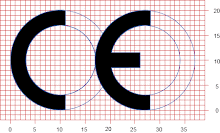
However, many appear to have overlooked the impact on industry of the proposed CE marking regime to RoHS.
If the proposals are adopted, which seems likely, RoHS compliance could well become a much more complex and resource sapping activity for everyone in the supply chain.
Many manufacturers and distributors have put processes in place to achieve compliance, but such procedures were of individual choice. Going forward, the proposals will mean a more uniform approach as manufacturers, importers and distributors follow the rules set out in the revised RoHS Directive.
Of great significance, the requirement to provide declarations of conformity in a standard format will be a huge shift from the mass of different certificates, statements and compliance documents that came with the existing RoHS legislation.
The proposed format is very clear and requires conformity with the RoHS Directive and gives no scope for qualifying statements such as "as far as we are aware" and such as "to the best of our knowledge" for example.
Manufacturers need to evaluate their processes, and those of any sub contractors, so they can provide the required declarations and apply the CE mark to their products. In turn, importers and distributors will have to check, and verify, what manufacturers have done.
The attached table outlines the proposed obligations for the manufacturer, importer and distributor.
Directive Decoder
SOURCE












.jpg)





.jpg)

.jpg)








.jpg)

.jpg)













.jpg)


.jpg)










































































































































































































.jpg)

.jpg)











.jpg)






















































.jpg)















.jpg)



























































































































.jpg)






















No comments:
Post a Comment